Designing spaces that foster brand love and help your business thrive.
Ask yourself: how can I attract more customers and boost my revenue?
If that's your question, then you're in the right place! We at Tanic Design can help you create a unique brand experience that will not only draw in customers, but also boost your revenue. Sounds good?
We help ambitious hospitality owners - with a hunger for growth to gain and retain customers with memorable and cohesive interior design.
You've always known you were made for more, and you're not afraid to go out there and make it happen. Get ready to experience the incredible potential life has to offer. It's time to turn your business into a thriving force of growth so you can live the life you've been daydreaming about non-stop.
How easy is it?
If you're serious about growing your business, we are here to make it happen.
Pick your package!
Ready to start? We've got three fantastic packages for you to choose from. Find the one that's just right for you and secure your spot!

Hop on a call with us!
Let's get the ball rolling! All we need is a quick chat to kick things off. And the best part? You can take this call from the comfort of your office or your go-to coffee shop.

We'll dive into your design strategy!
We'll jump right into crafting your design strategy. It's like preparing a roadmap, custom-made just for you, leading straight to success.
Designs you'll ❤️, guaranteed
GET YOUR unfair COMPETITIVE ADVANTAGE
Our design philosophy is all about creating spaces that not only look amazing but also evoke emotions and tell a story. The results? Your guests won’t just have a one-time fling with your brand – they’ll fall madly in love and form a lifelong connection. Tanic’s approach to design ensures that every aspect of your space is meticulously thought out, from the materials to the furniture selection and everything in between. And the best part? You’ll see tangible results that go far beyond aesthetics, like increased guest satisfaction and loyalty, and ultimately, higher revenue for your business.

Don't know how to start?
That's totally fine. We're on hand to navigate you through the whole process. From the first lightbulb moment to the final high-five, we're your sidekicks, making sure you're completely at ease and absolutely smitten with your brand and your new business.
Designs you'll ❤️, guaranteed
Now that we've spent some time together. Lemme ask... You are?
There's never been a better time to unlock the full potential of your business. Whether you're a hotel, restaurant, or coffee shop business, our plans are designed to cater to all your needs.
Recent work
Award winning designs, and nothing less.
Sur Villas
Find sanctuary on a rare stretch of land on Sur coast, enriched by nature and culture.

Al Qurashi Hotel
The Al Qurashi is designed to create a unique experience for pilgrims for their blessed journey to Mecca.

Private vacation complex
Case Study coming soon
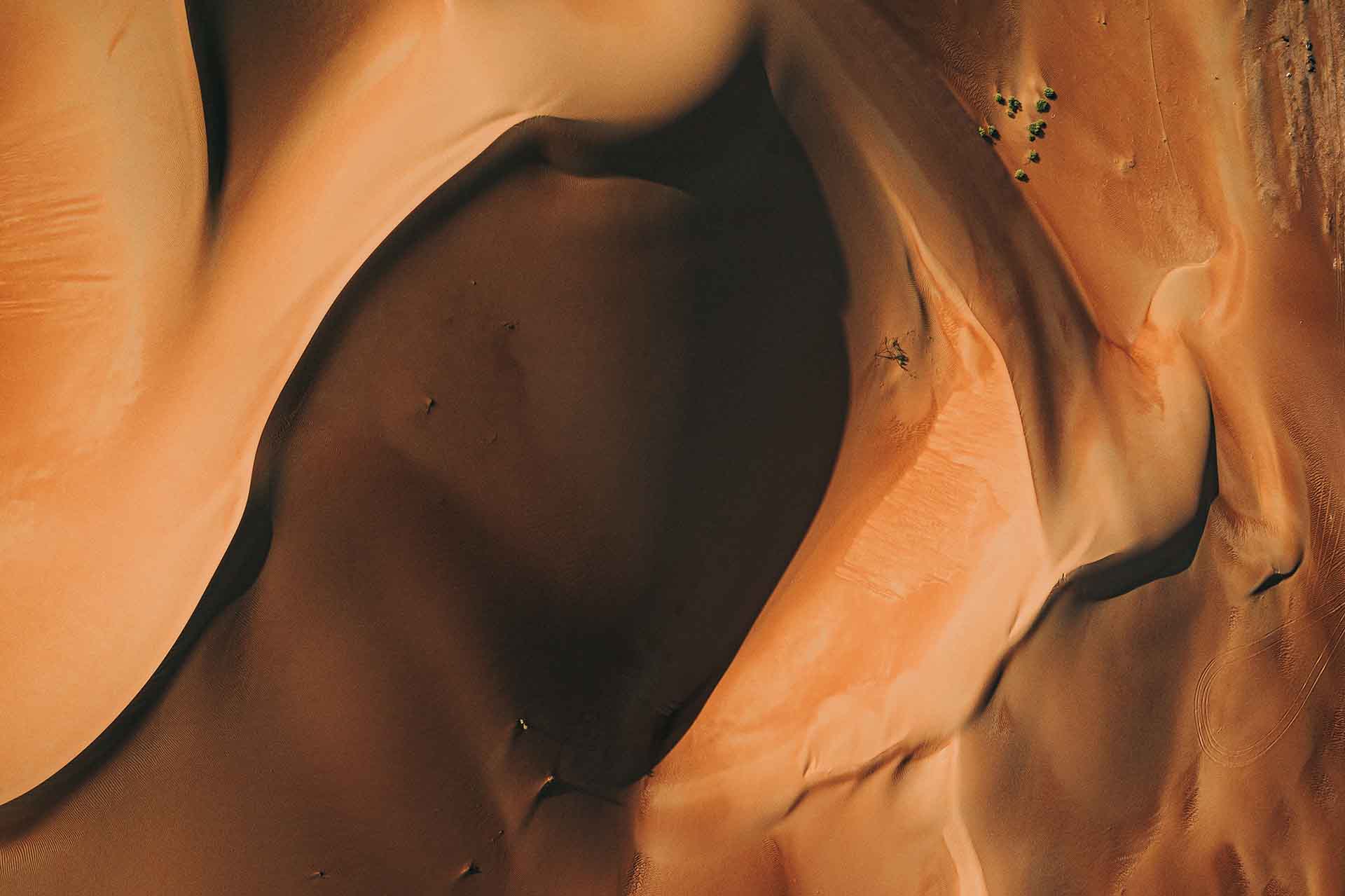
Coconut Beach
Coconut Cafe is a fresh and original luxury beach club concept located in The Move, the new high end mixed-use commercial project in Jubail.

Notorious Cafe
Inspired by 70’s design, Notorious is an immersive and exclusive experience in the form of a high-end cafe.

Spark
A feminine concept for a traditionally men-exclusive brand of gyms through the Middle East.

APPLICATION ONLY

We're not for everyone...
In order to maintain our unrivalled success-rate, we only accept a limited number of project at a time. Click below to apply to be in our next intake.

What our clients say

Melissa Waters
Professional, efficient, and delivered outstanding results. Couldn't be happier with their work!

Melissa Waters
Professional, efficient, and delivered outstanding results. Couldn't be happier with their work!

Melissa Waters
Professional, efficient, and delivered outstanding results. Couldn't be happier with their work!

Melissa Waters
Professional, efficient, and delivered outstanding results. Couldn't be happier with their work!

Melissa Waters
Professional, efficient, and delivered outstanding results. Couldn't be happier with their work!

Melissa Waters
Professional, efficient, and delivered outstanding results. Couldn't be happier with their work!

Melissa Waters
Professional, efficient, and delivered outstanding results. Couldn't be happier with their work!

Melissa Waters
Professional, efficient, and delivered outstanding results. Couldn't be happier with their work!

Melissa Waters
Professional, efficient, and delivered outstanding results. Couldn't be happier with their work!

Melissa Waters
Professional, efficient, and delivered outstanding results. Couldn't be happier with their work!

Melissa Waters
Professional, efficient, and delivered outstanding results. Couldn't be happier with their work!

Melissa Waters
Professional, efficient, and delivered outstanding results. Couldn't be happier with their work!

Melissa Waters
Professional, efficient, and delivered outstanding results. Couldn't be happier with their work!

Melissa Waters
Professional, efficient, and delivered outstanding results. Couldn't be happier with their work!

Melissa Waters
Professional, efficient, and delivered outstanding results. Couldn't be happier with their work!

Melissa Waters
Professional, efficient, and delivered outstanding results. Couldn't be happier with their work!

Melissa Waters
Professional, efficient, and delivered outstanding results. Couldn't be happier with their work!

Melissa Waters
Professional, efficient, and delivered outstanding results. Couldn't be happier with their work!

Melissa Waters
Professional, efficient, and delivered outstanding results. Couldn't be happier with their work!

Melissa Waters
Professional, efficient, and delivered outstanding results. Couldn't be happier with their work!
FAQs
How do I know that I will get a return on my investment?
Why is Tanic Design the best interior design consultancy?
Why does your process take such a long time?

Can you do it faster?

I have more questions about Tanic, how can I contact you?

I don't have a hospitality business, can we still work together?


How we scale hospitality brands to leading businesses in their area
We use our design magic to transform your business into a local hotspot. We'll craft spaces that are not only eye-catching but also a customer magnet.WHAT SETS THE BEST BUSINESSES APART FROM THE REST
Alright, listen up hospitality business owners. Feeling lost and unsure about where your customers are hiding? Wondering if they’ll ever come back after their first visit? It’s enough to make anyone feel like throwing in the towel. But hold on, before you start counting your losses and giving up on your dream, let me tell you this: there’s a better way. Say goodbye to the constant guessing game and the never-ending stress of trying to figure everything out yourself. Instead, why not make your business thrive with ease? But attracting customers isn’t just about putting up a sign and waiting for them to show up. Your potential clients are looking for more than just a place to eat or sleep, they want an experience, a connection. No need to reinvent the wheel here. Let us help you take your hospitality business to the next level, without all the headaches and sleepless nights. So, are you ready to ditch the uncertainty and start seeing some real results? Let’s do this.Designs you'll ❤️, guaranteed
Your first call for kickass hospitality projects

320%
Revenue growth increased. You'll notice a nice uptick in your revenue after joining forces with us.

4.9
Our clients are more than just satisfied, they're extra happy! Just check out our average rating from over 100 glowing reviews.

270%
This is just a fancy way of saying we check how much profit you're making from the money you've put into our services.

8 of 10
Our client retention rate? It's sky-high! Many clients loved working with us so much on the first project, they just had to come back for more.
HIGH-END INTERIOR DESIGN SERVICES
Would not be you looking for high-end residential interior design services?
Yup! This is the right place.
Hold up, don't go just yet!
Seems you need some more time to weight the options, and that's totally cool. Why not we hang out together?
© Tanic Design 2024. All Rights Reserved


